臭氧催化剂催化氧化降解盐酸四环素研究
摘要:四环素是一种常见抗生素,近些年来在地表水中被频繁检出,严重威胁水体安全及人类健康.采用水热法及浸渍焙烧法制备了Fe3O4-CeOx/AC催化剂,考察了利用该催化剂在臭氧氧化水中四环素中的性能,结果表明:在煅烧温度为600 ℃,煅烧时间为3 h,制备浸渍液中Fe3O4、Ce(NO3)3·6H2O、活性炭质量比为2∶1∶2的条件下,该催化剂效果很好,且持续实验90 min后仍可保持催化活性和较低的金属浸出率.当四环素初始浓度为20 mg·L-1,臭氧用量为4 mg·L-1·min-1,溶液初始pH为5,催化剂投加量为0.2 g·L-1时,10 min内四环素去除率可达大部分以上,90min内TOC去除率达到38%.利用SEM、XPS等多种手段对该催化剂进行了表征,通过淬灭实验证实在体系中·OH起到了催化作用.
1 引言(Introduction)
近年来,抗生素已被广泛应用于医疗行业,在人类和动物疾病防治等方面发挥了极大的作用(丁丽丹等,2021).抗生素是一类由细菌、霉菌等微生物代谢产生或人工合成的能够杀灭其他微生物的化学物质(张玮玮等,2013),按化学结构的不同可分为四环素类、多肽类、大环内脂类等(Bu et al.,2016).其中,四环素(tetracycline,简写为TC)是世界上应用很广泛的抗生素之一(Martinez,2009),因其难生物降解性,常常在地表水、土壤和地下水中被检测到(蒋海燕等,2020),对生态环境和人类健康造成严重的威胁(韩爽等,2021).
近年来基于臭氧、Fenton、过硫酸盐等的各种高级氧化技术已逐渐成为水污染控制领域的研究热点.其中,臭氧催化氧化反应快速高效且臭氧分解产物清洁,被视为一种极具潜力的废水处理方法.臭氧是一种非常活泼的气体,与氧气相比,它更容易溶于水.溶解后的臭氧以两种不同的方式与污染物发生反应,即臭氧分子可以直接选择性降解有机物,或通过生成二次氧化剂,如羟基自由基等非选择性降解有机物(Malik et al.,2019).由于臭氧的制造成本较高,其实际应用受到了限制,而引入催化剂的臭氧催化氧化技术大大提高了臭氧利用率,因此被广泛应用于工业水处理领域(任斌等,2020).一般情况下,催化臭氧化过程通常使用金属离子或金属氧化物作为催化剂(Wang et al.,2020).Chen等(2019)制备出的Fe3O4/Co3O4复合材料通过催化臭氧化系统显著提高磺胺甲恶唑的矿化效果,达到了60%的TOC去除率.但是金属氧化物作为催化剂单独使用也会出现活性位点不足,比表面积有限等缺点.将金属或金属氧化物负载于载体上,载体表面提供大量活性位点,从而表现出更高的活性(Yang et al.,2016).金属成分在赋予基体优异催化性能的同时,不可避免会出现金属浸出问题(李凯等,2021),因此,需要制备出一个具有较高催化活性且良好稳定性的催化剂.彭娟等将Mn、Fe、Ce 3种金属负载于活性炭(Activated Charcoal,简写为AC)上,使用该催化剂臭氧催化氧化120min去除了制药废水中80%的CODCr(彭娟等,2019).Li等用Fe-MCM-48催化剂结合O3处理双氯芬酸,60 min时TOC的去除率约为49.9%,是单一臭氧处理的2.0倍(Li et al.,2018).
在负载金属选择上,铁是一种十分常见的催化剂,铁不仅可以在臭氧催化体系中表现出较高的催化活性,还因其在自然界中含量丰富,廉价易得且易于合成(靳志豪等,2021),受到了越来越多的关注.此外,一些铁化合物具有特殊的特性,如Fe3O4具有磁性.近年来,Fe3O4纳米颗粒由于其低毒性、高磁性、高电性和可循环利用等优点(Zhang et al.,2020),作为催化剂受到越来越多的关注.Wu等制备的Fe3O4@SiO2@Mg(OH)2在臭氧催化氧化磺胺噻唑时,10 min内可去除99.0%以上的TOC,60 min内可去除40.1%(Wu et al.,2020).
本研究采用水热法与浸渍焙烧法制备了负载型Fe3O4-CeOx/AC磁性催化剂,并对实验条件进行了优化,通过SEM、XPS等分析手段表征了催化剂的形貌特征和结构组成,探究了Fe3O4-CeOx/AC臭氧催化氧化降解水体中四环素的效果及矿化程度,证明了非均相臭氧催化氧化降解抗生素的可行性.
2 材料与方法(Materials and methods)
2.1 实验试剂
六水氯化铁、颗粒活性炭购于上海麦克林生化科技有限公司,无水醋酸钠、聚乙二醇购于北京酷莱博科技有限公司,乙二醇、六水合硝酸铈、硫代硫酸钠、无水乙醇、甲醇(色谱级)、甲酸(色谱级)、盐酸四环素购于中国阿拉丁控股集团有限公司.
2.2 催化剂的制备
将颗粒型活性炭反复清洗烘干后研磨至粉末状,过100目筛留用.采用水热法制备Fe3O4:取0.65 g六水氯化铁、1.8 g醋酸钠、0.5 g聚乙二醇溶于20 mL乙二醇中,磁力搅拌1 h后,移至高压反应釜中在200 ℃下保持10 h,待完全冷却后取出,将反应后产物过0.22 μm水系滤膜,用乙醇和纯水交替冲洗2~3次,烘干后得到黑色Fe3O4粉末,可用磁铁检测其磁性.采用浸渍焙烧法制备Fe3O4-CeOx/AC复合催化剂:分别取一定量的硝酸铈、四氧化三铁和活性炭粉末溶解于纯水中,将混合溶液分别超声1 h、静置浸渍12 h后过膜并置于65 ℃烘箱中烘干12 h,将烘干后所得粉末置于管式炉中,在特定气氛保护下以5 ℃·min-1升温至600 ℃并保持3 h.待其冷却后取出,用乙醇清洗数次除去催化剂表面灰分并烘干备用.
2.3 催化氧化实验
本实验在室温下进行,采用1 L反应器,其中加入一定量催化剂与1 L浓度为20 mg·L-1的TC溶液.臭氧曝气头安装在反应器底部,接入由纯氧通过
臭氧发生器产生的臭氧.实验过程中为保证催化剂均匀分布,在上方放置机械搅拌器持续搅拌.每隔一定时间取适量溶液过0.22 μm水系膜放入液相小瓶,加入0.1 mL硫代硫酸钠溶液(0.1 mol·L-1)淬灭剩余臭氧,随后进行水质测定.
2.4 催化剂表征
使用扫描电子显微镜(SEM,TESCAN MIRA4,捷克)观察催化剂的微观形貌;使用全自动快速比表面与孔隙度分析仪(BET,麦克2460,美国)测定催化剂的比表面积;催化剂的表面元素组成和化学价态使用X射线光电子能谱仪(XPS,Thermo Scientific K-Alpha,美国)进行分析.
2.5 分析方法
TC浓度用岛津公司LC-16高效液相色谱仪进行测定,色谱柱为WondaSil C18-WR(4.6 mm×150 mm,5μm),流动相为25%甲醇和75%甲酸溶液(0.5%),流速为1 L·min-1,柱温为35 ℃,紫外检测器检测波长为355 nm;TOC 采用日本岛津公司TOC-L;溶液中浸出金属离子浓度采用美国安捷伦科技有限公司ICPOES730检测,更低检出限为0.01 mg·L-1.
3 结果与讨论(Results and discussion)
3.1 催化剂表征结果分析
3.1.1 催化剂形貌分析图1为催化剂AC及Fe3O4-CeOx/AC在不同标尺下的SEM图.从图1a~1c可以看出,负载前的AC在电镜下呈现出疏松多孔结构,孔壁光滑,孔道彼此交错贯通,具有较大的比表面积和孔体积,能提供较多负载位点;图1d~1f呈现了催化剂Fe3O4-CeOx/AC的形貌,可以明显看出在AC的孔道内部及表面上都存在大量球状的负载物,表面光滑且粒径大小均匀.图2的mapping图像中观察到,孔隙部分Fe分布均匀,表现明显,而Ce的表现不明显;根据图2e可以看出Ce在EDS能谱中表现出微弱的峰,说明催化剂上负载了铈,但其负载量较低,基本可以判断球状负载物主要为Fe3O4.以上结果可说明活性组分负载成功.
图1 AC(a~c)及Fe3O4-CeOx/AC(d~f)的SEM 图
通过BET测试得到了载体AC及经过负载的催化剂Fe3O4-CeOx/AC的比表面积和总孔体积.表1表明,经过铁和铈负载后的AC比表面积和总孔体积都比原先减少了近一半,由SEM结果可以知道,这是由于大量的负载物与AC结合,占据了原先的AC内部孔道和表面孔隙,因此催化剂Fe3O4-CeOx/AC的比表面积和总孔体积都远低于负载前的AC,这也说明了活性组分成功负载.
3.1.2 催化剂结构分析催化剂Fe3O4-CeOx/AC的C 1s、Fe 2p和Ce 3d的XPS图谱如下:通过XPS进一步分析催化剂的表面元素组成和化学价态,图3a 展示了C 1s 的谱图,位于284.8 eV 处有一个明显的主峰(Peak1),对应C-C键,此处对应于sp2 杂化的石墨C原子(刘创创等,2021),286.3和289.2 eV处的特征峰(Peak2/3)分别对应C-O和C=O键(黎素等,2021).
图2 催化剂中O(a)、C(b)、Fe(c)、Ce(d)的Mapping 图及EDS 谱图
图3b为Fe 2p的谱图,在728.1和724.6 eV 处的特征峰(Peak4/5)对应于Fe 2p1/2(Li et al.,2020;黎素等,2021),由Fe(II)氧化物和Fe(III)氧化物形成(Shwan et al.,2015);714.7和710.9 eV 处的特征峰(Peak1/2)分别对应Fe2+卫星峰和Fe 2p3/2(Yamashita et al.,2008);Fe 2p谱中的Fe 2p3/2和Fe 2p1/2的结合能峰值分别为710.9和724.6 eV,这两个峰的之间的距离大于13 eV,证明催化剂中存在Fe3+(Ma et al.,2016;Yu et al.,2019);同时在结合能为718.8和732.7 eV处也获得明显卫星峰(Peak3/6),进一步证实材料中有Fe3+(张帆等,2021).因此,复合催化剂中的铁的存在形态是Fe(II)和Fe(III)的氧化物.根据XPS图各电位峰面积统计,在催化剂的铁元素中,Fe(II)和Fe(III)的比例为0.58∶0.42,接近于1∶1.催化剂制备原料之一为Fe3O4,理论上Fe(II)和Fe(III)的比例应为1∶2,说明催化层中Fe的价态在制备过程中发生了变化,有一部分Fe(III)被还原为Fe(II),这是还原性的制备环境所致.催化层在与臭氧分子作用,产生活性自由基过程中,涉及到电子转移,电子越容易转移,生成的自由基越多.推测Fe(II)和Fe(III)接近1∶1的价态比例有利于电子转移和活性自由基的生成.
图3c为Ce 3d的谱图,在882.1~898.1和900.5~916.5 eV处的结合能对应于Ce 3d 5/2和Ce 3d 3/2(Li etal.,2020). 其中,886.9、897.9 和904.4 eV 处特征峰(Peak1/3/5)对应Ce(III)氧化物;888.2、901.2、907.0 和916.6 eV的峰(Peak2/4/6/8)对应于Ce(IV)氧化物相关(He et al.,2018 ;Wu et al.,2018),这可能是由于催化剂在煅烧制备过程中Ce(III)发生了氧化,也可能是在烘干和保存过程中一部分Ce(III)被氧化(Sun et al.,2019).其中,Ce(III)和Ce(IV)比例约为0.49∶0.51.
表2显示了催化剂中主要组成成分,包括C以及不同价态的金属离子所占的实际比例.可以看出Fe3O4-CeOx/AC中初始的铁离子、铈离子占比分别为27.35%、3.24%,其中铈离子占比较低与SEM 表征结果一致,推测该种情况出现的原因可能是以浸渍法在活性炭上负载金属铈的过程中,有较大部分的铈依旧存在于浸渍液中或仅仅吸附于载体表面,在催化剂制备过程中的清洗等阶段流失,仅有一部分的铈成功负载,并更终在焙烧下以不同价态氧化物形式固定于载体活性炭上.通过XPS的分析结果进一步证明了铁铈成功与活性炭复合.
图3 催化剂Fe3O4-CeOx/AC 的XPS 图
3.2 催化氧化实验结果及分析
通过催化氧化实验,得到不同催化体系、催化剂组成配比、pH及催化剂投加量对TC降解效果的影响如下.
3.2.1 不同催化体系差异图4a展示了不同催化体系对TC催化降解效果的影响,反应条件:TC浓度为20mg·L-1,溶液初始pH为5.8,催化剂投加量为0.2 g·L-1,臭氧用量为4 mg·L-1·min-1.可以看出,单独O3、O3+AC、O3+CeOx/AC、O3+Fe3O4/AC、O3+Fe3O4-CeOx/AC不同实验体系下10 min内,TC去除率分别为94.38%、96.77%、98.23%、99.41%、99.94%,反应速率常数分别为0.288、0.346、0.402、0.507、0.710 min-1(R2=0.996/0.992/0.999/0.994/0.970).可以看出,相较于单臭氧体系,加入催化剂后TC的降解效率有了明显提升,其中在O3+Fe3O4-CeOx/AC的实验体系下,TC降解效果更好.此外在O2+ Fe3O4-CeOx/AC体系中TC去除率仅为7%,说明Fe3O4-CeOx/AC吸附去除TC能力较低,主要通过催化臭氧氧化来去除溶液中TC.
3.2.2 催化剂组成配比的影响图4b展示了不同催化剂组成配比对TC催化氧化效果的影响,其他反应条件与先前保持一致.分别调整浸渍液中Fe3O4、Ce(NO3)3·6H2O和活性炭质量比例,可以看出,当浸渍液中Fe3O4质量不变时,提高硝酸铈和活性炭的质量,催化剂的效果差异不大,但当在催化剂体系中Fe3O4的质量增加时,催化速率有所提升,通过SEM和XPS的结果也可以看出以浸渍法负载的铈含量较低,相较于Fe3O4所起到的作用较少.由此可以推测在该催化体系中,Fe3O4是主要活性成分,能起到主要的催化作用.根据实验结果确定制备催化剂浸渍液中各组分质量比为Fe3O4∶Ce(NO3)3·6H2O∶AC=2∶1∶2.根据XPS表征结果确定更终方案中催化剂各组分的实际组成配比约为铁离子∶铈离子∶活性炭=9∶1∶23.
3.2.3 pH 值的影响图4c展示了实验体系初始pH对TC催化降解效果的影响,其他反应条件与先前保持一致.不难发现,实验体系在酸性及中性条件下催化效果明显好于碱性,其中,当pH为5时效果更佳.说明适量H+的存在对催化氧化过程具有一定的促进作用,这对后面的该催化剂的催化机理探讨具有重要启示作用.
3.2.4 催化剂投加量的影响图4d展示了不同催化剂投加量对TC催化降解效果的影响,其他反应条件与先前保持一致.可以看出,将催化剂投加量从0.05 g·L-1逐渐增加到0.2 g·L-1,催化效果逐渐提升;当再增加催化剂的投加量,催化效果没有明显提升,当催化剂投加量增加至0.8 g·L-1时,催化效果反而下降,可能原因为过量催化剂的加入,分散程度有限(Zhang et al.,2021),催化剂上活性位点也有限(张万鹏等,2020),另一方面当催化剂浓度较高时,产生的自由基相互间会发生淬灭(许光益等,2018),从而影响TC的降解效果.因此在充分考虑实验效果及资源合理利用的前提下,将催化剂投加量确定为0.2 g·L-1.
图4 不同催化体系(a),催化剂组成配比(b),pH(c)及催化剂投加量(d)对TC 降解效果的影响
3.2.5 矿化效果通过测定TOC去除率对催化剂Fe3O4-CeOx/AC催化臭氧氧化TC的矿化效果进行了评价.
从图5可见,单独臭氧氧化90 min时,TOC去除率仅为25%,而经过催化剂Fe3O4-CeOx/AC催化臭氧化90 min时,TOC的去除率达到了38%,相较于单独臭氧氧化大大提升,这也说明,催化剂Fe3O4-CeOx/AC存在时会促进TC的矿化.
图5 O3及Fe3O4-CeOx/AC+O3体系的TOC 去除率 图6 Fe3O4-CeOx/AC 催化剂稳定性探究
3.3 催化剂稳定性
3.3.1 稳定性实验将催化剂置于实验体系中持续实验90 min以上,取使用后催化剂再次实验(记为Fe3O4-CeOx/AC'),与首次使用的催化剂催化效果对比如图6所示.可以看出,在实验体系中连续运行90 min后的催化剂催化效果并无明显降低,证明催化剂有较好的稳定性,长时间使用后仍保持较好的催化性能.
3.3.2 金属浸出浓度将催化剂置于实验体系中持续实验90 min以上,取实验后溶液对其中金属离子浓度进行测定,判断催化剂的金属浸出情况.ICP 检测结果如表3所示,90 min 后溶液中铁离子浓度为0.0608mg·L-1,铈离子浓度为0.0290 mg·L-1,可以看出催化剂的金属浸出较少,说明其稳定性较好.
3.4 降解机理探究
3.4.1 淬灭实验为探究臭氧催化氧化降解TC的作用机理,验证实验体系中自由基的存在,以NaHCO3和对苯醌(p-benzoquinone,简写为BQ)作为淬灭剂.向实验体系中分别加入5 mmol NaHCO3和BQ,结果如图7所示.
结果表明,加入NaHCO3 后,Fe3O4-CeOx/AC+O3催化体系呈现出抑制效果:在O3+Fe3O4-CeOx/AC催化体系下10 min内,TC去除率为99.94%,反应速率常数为0.710 min-1(R2=0.970),而在加入NaHCO3 后,10 min 内TC 去除率为96.6%,反应速率常数降为0.331 min-1(R2=0.997),很大程度上影响了催化效果和催化速率,也证实了体系中·OH的存在.而加入BQ并未对Fe3O4-CeOx/AC+O3催化体系的降解效果产生较为明显的影响,根据此结果可得出·O2-并不是该体系中主要起到催化作用的活性自由基.综上所述,·OH在该实验体系起到了重要的催化作用.
图7 不同淬灭剂对实验体系TC 降解效果的影响(a)及反应速率常数(b)
3.4.2 降解机理讨论目前在对于臭氧催化氧化技术作用机理上仍有不同看法,但大多数学者支持自由基理论.由淬灭实验可知,NaHCO3的加入大大影响了催化效果,NaHCO3淬灭·OH的公式见式(1)(Afzal et al.,2017).
HCO3− + ·OH → CO3·− + H2O K•OH = 8.5 × 106 L·mol−1·s−1 (1)
因此可以基本判断实验体系中存在·OH.金属催化臭氧分解产生·OH的机理用方程表示为式(2)(Wanget al.,2020).
Mn + + O3 + H+ → M(n + 1) + + ·OH + O2 (2)
由此可以说明Fe(II)和Fe(III)、Ce(III)和Ce(IV)两对氧化-还原体系的循环在促进·OH的形成中起到了重要作用(李民等,2017;Xiong et al.,2019),同时也对应前面pH的影响试验结果,证明H+在催化氧化过程中存在的必要性.
在XPS的表征结果下还可以看出直接以Fe3O4形式去负载活性炭所制备出的催化剂,理论上Fe(II)和Fe(III)的比例应为1∶2,但实际测得Fe(II)和Fe(III)的比例约为1∶1,Fe(II)占比远高于理论值.由于催化剂制备在特殊的高温还原性气氛中进行,制备环境中氧分子不足,使金属氧化物晶格中的氧原子逸出,由此产生较多的氧空位,催化剂整体带正电荷,是关键的催化活性位点.铁和铈都具有两种价态,且其比例在制备的催化剂中均接近于1∶1,有利于电子转移.氧空位数量的增加和电子转移能力的提高均非常有利于生成更多的·OH,从而表现出较优的催化能力.
催化层中存在大量的氧空位和适宜比例的Fe(II)和Fe(III)、Ce(III)和Ce(IV)两对氧化-还原体系,对于催化过程中增加催化活性位点数量和提高电子转移能力,从而促进·OH的生成具有重要意义.氧空位数量和氧化-还原体系是提高催化剂性能的关键影响因素(Wang et al.,2019),富含氧空位并且能够促进电子转移和O3离解是催化剂展示出优异催化性能的主要原因(Guo et al.,2019).关于该催化剂性能有待进一步的深入研究.
据此,可以将Fe3O4-CeOx/AC的催化活性归因于以下因素:首先是催化剂具有较大的比表面积(Chen etal.,2019);其次,具有丰富的氧空位作为与O3作用的催化点位;再次,特殊的制备条件得到了适宜比例的Fe(II)和Fe(III)、Ce(III)和Ce(IV)活性层,增强了电子传递能力,有利于催化促进·OH等强氧化剂的产生(Park et al.,2003).此外,在水溶液中,金属氧化物表面容易通过水分子的解离性化学吸附而发生羟基化(Tamura et al.,1999).这些羟基可以释放质子,充当Brönsted酸位.同时,当吸附的水分子被解吸时,会形成金属阳离子和配位不饱和氧,分别充当路易斯酸和路易斯碱位,金属氧化物的表面羟基和这些位点都可以有效提升催化效率(Wang et al.,2017).
4 结论(Conclusions)
1)利用水热法制备出的Fe3O4与硝酸铈、活性炭粉末按一定比例在溶液中混合均匀并浸渍12 h后在管式炉中600 ℃煅烧3 h后得到Fe3O4-CeOx/AC复合材料,在TC初始浓度为20 mg·L-1,臭氧用量为4 mg·L-1·min-1,溶液初始pH为5,催化剂投加量为0.2 g·L-1时,10 min内TC去除率可达大部分以上,90 min内TOC去除率达到38%.
2)从SEM结果可以看出,催化剂Fe3O4-CeOx/AC可以明显看出在AC的孔道内部及表面上都存在大量球状的负载物;BET测试表明了经过铁和铈负载后的催化剂比表面积和总孔体积都有较大变化;XPS谱图显示催化剂主要由C、O、Fe、Ce 4种元素组成,其中Fe和Ce都具有多价态及其氧化物形态.以上结果可说明活性组分负载成功.
3)通过淬灭实验证明了体系中产生了·OH:在加入NaHCO3后,O3+Fe3O4-CeOx/AC催化体系10 min内TC去除率由99.94%降至96.6%,反应速率常数为0.710 min-1降为0.331 min-1,明显抑制了TC的降解.在加入BQ后,降解效果则并没有收到太大影响.因此说明在实验体系中存在·OH,且·OH起到了一定的催化作用,·O2-则并未在该体系中表现出明显催化效果.
参考文献(References):
Afzal S,Quan X,Zhang J L. 2017. High surface area mesoporous nanocast LaMO3(M=Mn,Fe)perovskites for efficient catalytic ozonation and an insight into probable catalytic mechanism [J]. Applied Catalysis B-Environmental, 206: 692-703
Bu Q W,Wang B,Huang J,et al. 2016. Estimating the use of antibiotics for humans across China [J]. Chemosphere, 144(2): 1384-1390
Chen H,Wang J L. 2019. Catalytic ozonation of sulfamethoxazole over Fe3O4/Co3O4 composites [J]. Chemosphere, 234: 14-24
Chen W R,Bao Y X,Li X K,et al. 2019b. Mineralization of salicylic acid via catalytic ozonation with Fe-Cu@SiO2 core-shell catalyst:A two-stage first order reaction [J]. Chemosphere, 235(11): 470-480
丁丽丹,周家斌,刘文博,等. 2021. CuO/Bi2O3光催化耦合过一硫酸盐氧化降解盐酸四环素[J]. 环境工程学报, 15(3): 898-910
Guo X X,Hu T T,Meng B,et al. 2019. Catalytic degradation of anthraquinones-containing H2O2 production effluent over layered Co-Cu hydroxides:Defects facilitating hydroxyl radicals generation [J]. Applied Catalysis B-Environmental, 260: 118157
韩爽,肖鹏飞. 2021. 过硫酸盐活化技术在四环素类抗生素降解中的应用进展[J]. 环境化学, 40(9) : 2873-2883
He S M,Luan P C,Mo L H,et al. 2018. Mineralization of recalcitrant organic pollutants in pulp and paper mill wastewaters through ozonation catalyzed by Cu-Ce supported on Al2O3 [J]. Bioresources, 13: 3686-3703
蒋海燕,段毅,刘宇琪,等. 2020. 煅烧高岭土活化过一硫酸盐去除废水中的四环素[J] . 环境工程学报, 14(9): 2494-2505
靳志豪,黄远星,付小洁,等. 2021. 新型铁铈复合氧化物催化臭氧氧化工艺去除对硝基苯酚[J]. 净水技术, 40(1): 88-95
Li J Y,Song W F,Mao X C,et al. 2020. Catalytic ozonation of dairy farming wastewater using a Mn-Fe-Ce/gamma-Al2O3 ternary catalyst:Performance,generation,and quenching of hydroxyl radicals [J]. Journal of Physical Chemistry C, 124: 13215-13224
李凯,刘新璐,李辉,等. 2021. 狐尾藻生物炭活化过硫酸盐降解四环素的研究[J]. 环境科学与技术, 44 (6) : 50-57
李民,陈炜鸣,蒋国斌,等. 2017. Fe-Ce/GAC催化臭氧降解高浓度腐殖酸废水[J]. 环境科学学报, 37(9): 3409-3418
黎素,张博,谢春生,等. 2021. Bi-FeC2O4复合催化剂活化过硫酸盐降解罗丹明B [J]. 环境科学学报, 41(7): 2796-2805
Li X K,Chen W R,Tang Y M,et al. 2018. Relationship between the structure of Fe-MCM-48 and its activity in catalytic ozonation for diclofenac mineralization [J]. Chemosphere, 206: 615-621
刘创创,王逸,周丽华,等. 2021. 纳米N-C/ZrO2活化过硫酸盐降解苯酚的机理研究[J]. 环境科学学报, 41(10): 3956-3968
Ma K Y,Cheng J P,Zhang J,et al. 2016. Dependence of Co/Fe ratios in Co-Fe layered double hydroxides on the structure and capacitive properties [J]. Electrochimica Acta, 198: 231-240
Malik S N,Khan S M,Ghosp P C,et al. 2019. Treatment of pharmaceutical industrial wastewater by nano-catalyzed ozonation in a semi-batch reactor for improved biodegradability [J]. Science of the Total Environment, 678: 114-122
Martinez J L. 2009. Environmental pollution by antibiotics and by antibiotic resistance determinants [J]. Environmental Pollution, 157: 2893-2902
Park C,Keane M A. 2003. Catalyst support effects:Gas-phase hydrogenation of phenol over palladium [J]. Journal of Colloid and Interface Science, 266: 183-194
彭娟,杨永哲,杨宏勃,等. 2019. Fe-Mn-Ce/GAC催化剂制备及其在生物制药废水深度处理中的应用[J]. 环境工程, 37(12): 113-119
任斌,金政伟,李瑞龙,等. 2020. 臭氧催化氧化降解煤化工高盐废水有机物研究[J]. 工业用水与废水, 51: 29-32+53
Shwan S,Jansson J,Olsson L,et al. 2015. Chemical deactivation of H-BEA and Fe-BEA as NH3-SCR catalysts-effect of potassium [J]. Applied Catalysis B-Environmental, 166: 277-286
Sun M L,Zhang W D,Sun Y J,et al. 2019. Synergistic integration of metallic Bi and defects on BiOI:Enhanced photocatalytic NO removal and conversion pathway [J]. Chinese Journal of Catalysis, 40: 826-836
Tamura H,Tanaka A,Mita K,et al. 1999. Surface hydroxyl site densities on metal oxides as a measure for the ion-exchange capacity [J]. Journal of Colloid and Interface Science, 209: 225-231
Wang J L,Bai Z Y. 2017. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater [J]. Chemical Engineering Journal, 312: 79-98
Wang J L,Chen H. 2020. Catalytic ozonation for water and wastewater treatment:Recent advances and perspective [J]. Science of the Total Environment, 704: 135249
Wang Y X,Chen L L,Cao H B,et al. 2019. Role of oxygen vacancies and Mn sites in hierarchical Mn2O3/LaMnO3-δ perovskite composites for aqueous organic pollutants decontamination [J]. Applied Catalysis B-Environmental, 245: 546-554
Wu J,Sun Q,Lu J. 2020. Synthesis of magnetic core-shell Fe3O4@SiO2@Mg(OH)2 composite using waste bischofite and its catalytic performance for ozonation of antibiotics [J]. Journal of Environmental Chemical Engineering, 8(5): 104318
Wu Z W,Zhang G Q,Zhang R Y,et al. 2018. Insights into mechanism of catalytic ozonation over practicable mesoporous Mn-CeOx/gamma-Al2O3 catalysts [J]. Industrial & Engineering Chemistry Research, 57: 1943-1953
Xiong W,Chen N,Feng C P,et al. 2019. Ozonation catalyzed by iron-and/or manganese-supported granular activated carbons for the treatment of phenol [J]. Environmental Science and Pollution Research, 26: 21022-21033
许光益,隋铭皓,袁博杰,等. 2018. 纳米CuFe2O4活化过一硫酸盐降解诺氟沙星性能[J]. 哈尔滨工业大学学报, 50(2): 46-53
Yamashita T,Hayes P. 2008. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials [J]. Applied Surface Science, 254: 2441-2449
Yang X J,Xu X M,Xu X C,et al. 2016. Modeling and kinetics study of Bisphenol A(BPA)degradation over an FeOCl/SiO2 Fenton-like catalyst [J]. Catalysis Today, 276: 85-96
Yu D Y,Wu M H,Hu Q,et al. 2019. Iron-based metal-organic frameworks as novel platforms for catalytic ozonation of organic pollutant:Efficiency and mechanism [J]. Journal of Hazardous Materials, 367: 456-464
张帆,宋阳,胡春,等. 2021. 铁钛共掺杂氧化铝诱发表面双反应中心催化臭氧化去除水中污染物[J]. 环境科学, 42(5): 2360-2369
Zhang H,He Y L,Lai L D,et al. 2020. Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4-MnO2 magnetic composites:Performance,transformation pathways and mechanism [J]. Separation And Purification Technology, 245:116449
Zhang L F,Zhang L H,Sun Y L,et al. 2021. Porous ZrO2 encapsulated perovskite composite oxide for organic pollutants removal:Enhanced catalytic efficiency and suppressed metal leaching [J]. Journal of Colloid and Interface Science, 596: 455-467
张万鹏,郑立庆,杨鑫雨,等. 2020. 球磨-煅烧法制备Fe3O4-CuxO及其活化Oxone降解盐酸左氧氟沙星[J]. 中国环境科学, 40(1): 143-152
张玮玮,弓爱君,邱丽娜,等. 2013. 废水中抗生素降解和去除方法的研究进展[J]. 中国抗生素杂志, 38: 401-410


 当前位置:
当前位置: 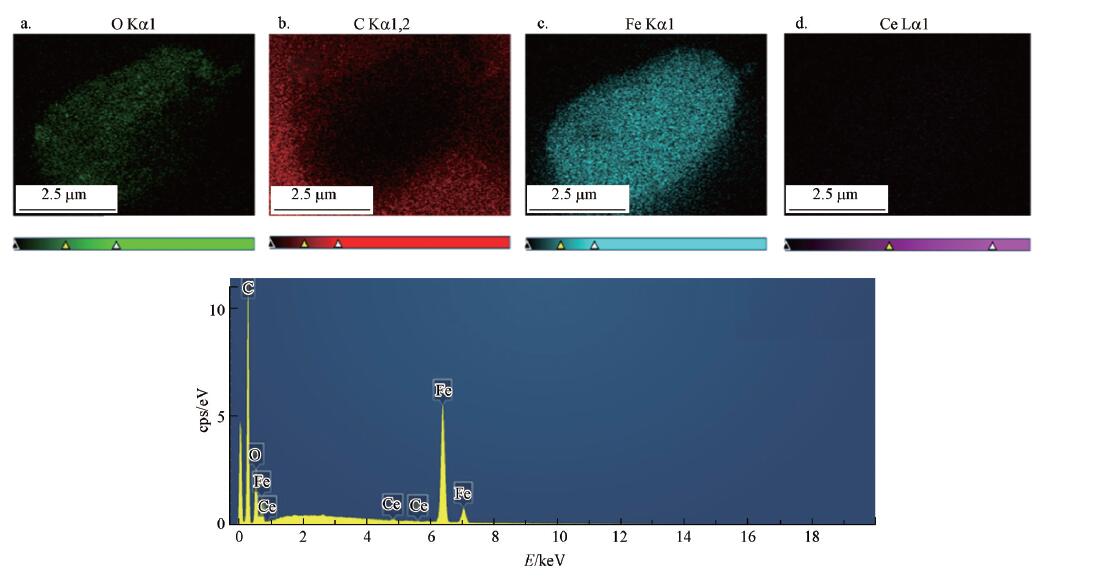
 摘要
摘要







 上一篇:
上一篇: 返回列表
返回列表
